Membrane protein organization studied at the single molecule level
Employing the unique capabilities of liquid phase electron microscopy (LP-EM) to image proteins in intact cells in their native liquid environment at the nanoscale, we study the biophysical principles of how a cell organizes its functional membrane proteins in specific locations and configurations in order to regulate function. We selected two membrane proteins, the ORAI1 protein, forming store-operated calcium channels, and HER2, member of the receptor tyrosine kinase family of epidermal growth factor receptors (EGFRs). ORAI1 was found to organize in elongated supramolecular clusters, in addition to the known assembly in larger clusters, so-called puncta, forming upon store operated Ca2+ entry (SOCE) activation, see: Int. J. Mol. Sci. 22, 799 (2021). HER2 accumulated together with actin in dynamic membrane areas such as membrane ruffles, and the leading edges of migrating cells, see: Front. Cell Dev. Biol. 8, 521 (2020). ORAI1 and HER2 proteins are both known to directly and indirectly control the dynamics of the actin cytoskeleton, the main component of the dynamic cellular structure responsible for cell migration. However, the underlying mechanisms, as well as their interdependencies are still not completely understood. Cell migration is crucial for many physiological processes that occur during development, as well as for a healthy immune response.
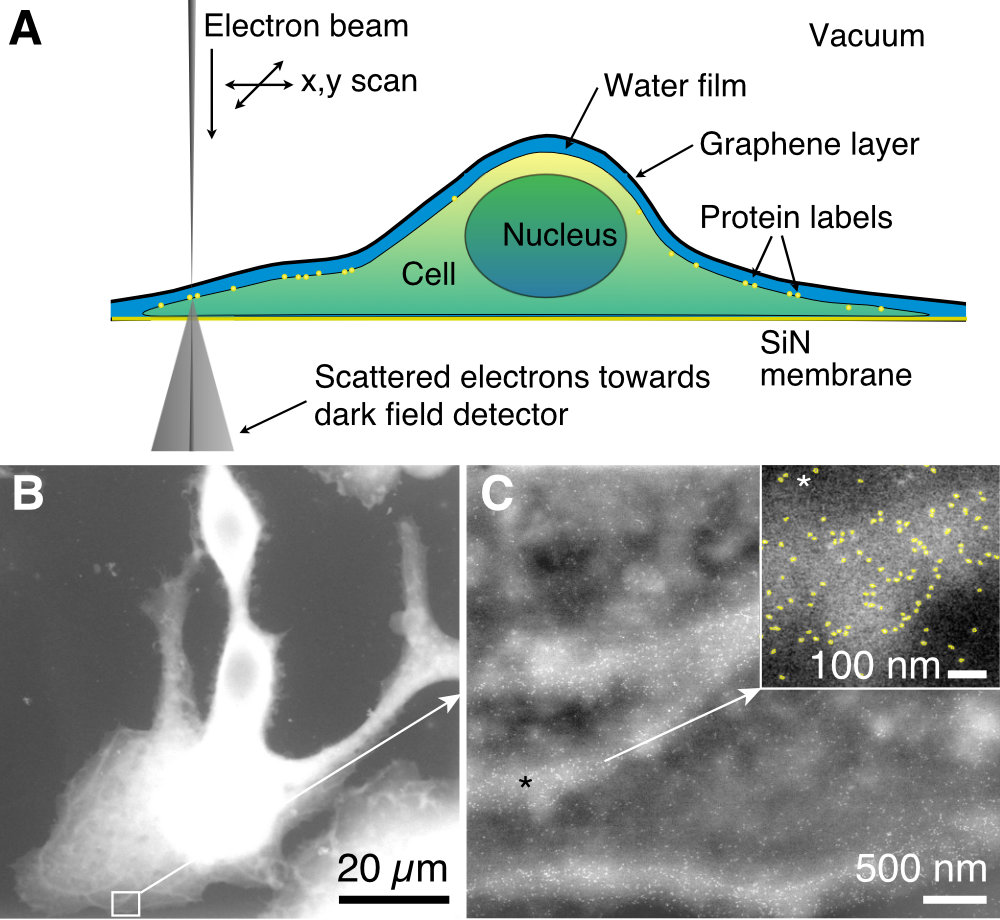
Figure at the left: LP-EM of a whole cell. (A) Scheme of a cell in a liquid layer covered by a graphene sheet on a thin SiN membrane imaged with a STEM electron beam. (B). Overview image of whole SKBR3 cancer cells in liquid. (C) Image of the boxed region in B showing individual HER2 proteins labeled with QDs at a spatial resolution of 3 nm. From: Biophys. J. 115, 503 (2018)
This research is conducted within the context of the DFG Collaborative Research Centre 1027 “Physical modeling of non-equilibrium processes in biological systems” together with the groups headed by Prof. Matthias Hannig, UkS, Prof. Barbara Niemeyer, UkS, Prof. Franziska Lautenschläger, UdS, and Prof. Arancha del Campo, INM.